SPEC Kit 334: Research Data Management Services · 177
JOHNS HOPKINS UNIVERSITY
Appendix V from Policy on Access and Retention of Research Data and Materials
http://jhuresearch.jhu.edu/DMP_AppendixFive.pdf
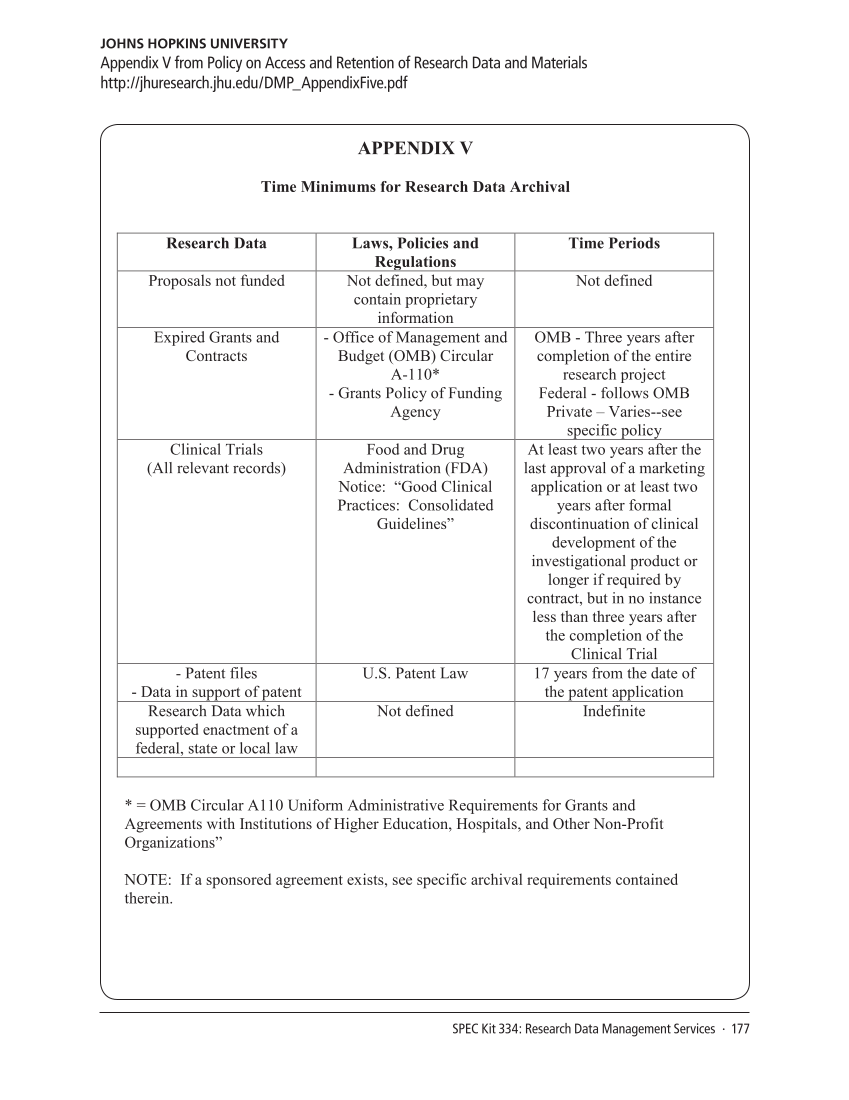
APPENDIX V
Time Minimums for Research Data Archival
Research Data Laws, Policies and
Regulations
Time Periods
Proposals not funded Not defined, but may
contain proprietary
information
Not defined
Expired Grants and
Contracts
-Office of Management and
Budget (OMB) Circular
A-110*
-Grants Policy of Funding
Agency
OMB -Three years after
completion of the entire
research project
Federal -follows OMB
Private – Varies--see
specific policy
Clinical Trials
(All relevant records)
Food and Drug
Administration (FDA)
Notice: “Good Clinical
Practices: Consolidated
Guidelines”
At least two years after the
last approval of a marketing
application or at least two
years after formal
discontinuation of clinical
development of the
investigational product or
longer if required by
contract, but in no instance
less than three years after
the completion of the
Clinical Trial
-Patent files
-Data in support of patent
U.S. Patent Law 17 years from the date of
the patent application
Research Data which
supported enactment of a
federal, state or local law
Not defined Indefinite
*=OMB Circular A110 Uniform Administrative Requirements for Grants and
Agreements with Institutions of Higher Education, Hospitals, and Other Non-Profit
Organizations”
NOTE: If a sponsored agreement exists, see specific archival requirements contained
therein.
JOHNS HOPKINS UNIVERSITY
Appendix V from Policy on Access and Retention of Research Data and Materials
http://jhuresearch.jhu.edu/DMP_AppendixFive.pdf
APPENDIX V
Time Minimums for Research Data Archival
Research Data Laws, Policies and
Regulations
Time Periods
Proposals not funded Not defined, but may
contain proprietary
information
Not defined
Expired Grants and
Contracts
-Office of Management and
Budget (OMB) Circular
A-110*
-Grants Policy of Funding
Agency
OMB -Three years after
completion of the entire
research project
Federal -follows OMB
Private – Varies--see
specific policy
Clinical Trials
(All relevant records)
Food and Drug
Administration (FDA)
Notice: “Good Clinical
Practices: Consolidated
Guidelines”
At least two years after the
last approval of a marketing
application or at least two
years after formal
discontinuation of clinical
development of the
investigational product or
longer if required by
contract, but in no instance
less than three years after
the completion of the
Clinical Trial
-Patent files
-Data in support of patent
U.S. Patent Law 17 years from the date of
the patent application
Research Data which
supported enactment of a
federal, state or local law
Not defined Indefinite
*=OMB Circular A110 Uniform Administrative Requirements for Grants and
Agreements with Institutions of Higher Education, Hospitals, and Other Non-Profit
Organizations”
NOTE: If a sponsored agreement exists, see specific archival requirements contained
therein.